![]() 301,
Building B, No. 2590 Nanhuan Road, Binjiang District, Hangzhou City, Zhejiang Province
301,
Building B, No. 2590 Nanhuan Road, Binjiang District, Hangzhou City, Zhejiang Province
![]() +86-13167003458
+86-13167003458
![]() marketing@jyssbio.com
marketing@jyssbio.com
More than 3 years after the outbreak of the COVID-19, the situation is still severe, but the proportion of asymptomatic infections in domestic confirmed cases has risen significantly to 92%-95%, which is related to the high vaccination rate in China. As of the latest report on April 13, China has reported a total of 3.35 billion doses of the new coronavirus vaccine, of which bioprocess membranes, as an important raw material for production, have played an indispensable role in the production of vaccines.
JYSS BIO is the first high-tech enterprise in China to realize the localization of the process technology of Single-use bioreactors and Single-use Assemblies. Since the outbreak of the COVID-19 epidemic, JYSS BIO has supplied Single-use bioreactors and Single-use Assemblies for well-known domestic vaccine manufacturers in the ongoing battle with the COVID-19 pandemic. Its EB 1596 film is the backbone of the fight against the COVID-19 epidemic, and it is also the first domestic bioprocessing film adopted in the commercial large-scale production in China.
Structure of EB1596 film
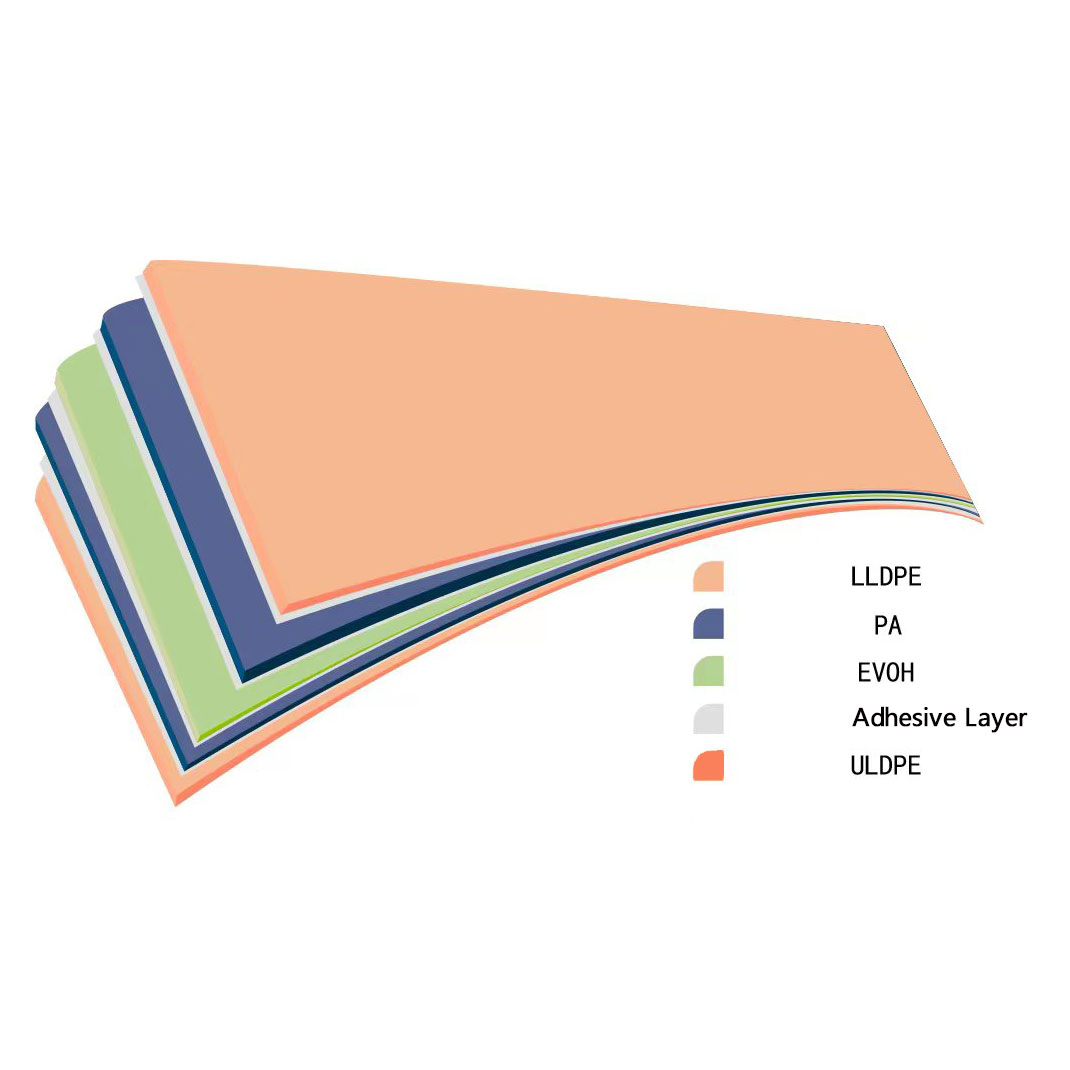
Outer layer, adhesive layer, material-liquid contact layer
l ULDPE ultra-low density polyethylene-material-liquid contact layer, using inert material transparent, strong, pressure-resistant, and very low E&L (extractables and leachables)
l EVOH ethylene-vinyl alcohol copolymer-gas barrier layer
l LLDPE linear low density polyethylene-the outermost film has stronger anti-friction, breaking ability and better tensile force
l PA nylon—compared to other membrane materials, EB1596 has two more layers of PA nylon, which makes the film more robust and puncture resistant.
l Adhesive layer along with LLDPE increases film strength and toughness at low temperature.
Physical and chemical performance Testing
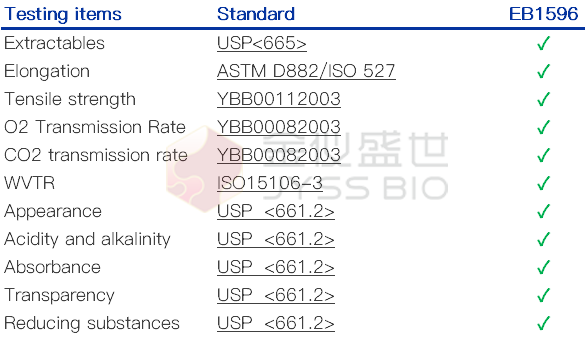
JYSS BIO EB1596 film has passed 11 physical and chemical performance tests, complying with USP <661>, <665>, ISO15106, YBB00082003, etc.
Biocompatibility Testing

In terms of biocompatibility, EB1596 bioprocess film has passed USP <85><87><88>, ISO 10993, etc. Total of 7 strict testing ensures more suitable environment for cell culture.
Cell Culture Performance
The cell culture in JYSS BIO Single-use bioreactors and bags achieved high-density and high-viability growth of cells, ensuring the yield of target products and the consistency between culture batches.
CHO-K1 cells cultured by EB1596 film
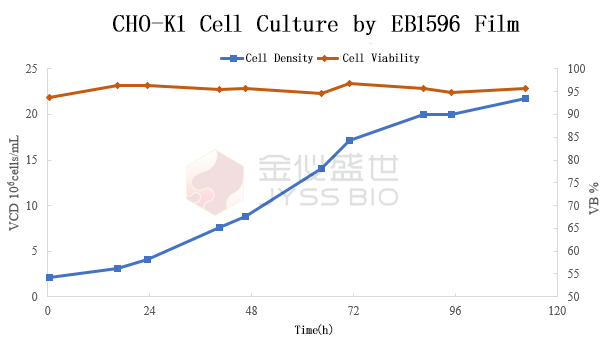
The above chart shows CHO-K1 cells culture in JYSS EB1596 bioprocess film, where the cell density reached a maximum value of 2.2×10⁷cells/mL after 120 hours.
Related Products

Products made of EB1596 bioprocess film include Single-use bioreactor bags, magnetic mixing bags, liquid storage bags, sampling bags, etc.