![]() 301,
Building B, No. 2590 Nanhuan Road, Binjiang District, Hangzhou City, Zhejiang Province
301,
Building B, No. 2590 Nanhuan Road, Binjiang District, Hangzhou City, Zhejiang Province
![]() +86-13167003458
+86-13167003458
![]() marketing@jyssbio.com
marketing@jyssbio.com
Recently, the U.S. Department of Commerce’s Bureau of Industry and Security (BIS) posted a new batch of “Unverified Lists (UVLs)” online. In this "Unverified List (UVL)", 33 Chinese companies have been added, and Chinese well-known CDMO companies are on the list, causing their stock prices to fluctuate violently.
This incident once again sounded the alarm for the development of China's pharmaceutical industry, and the bottlenecks that affect the independent development of the industry are in urgent need of breakthroughs. The stable and controllable industrial chain and supply chain is one of the six specific goals of the "14th Five-Year Plan" for the pharmaceutical industry. The plan proposes that the advantages of large-scale and systematic pharmaceutical manufacturing will be further consolidated, a number of key common technologies for industrialization have made breakthroughs, and positive results have been achieved in key areas to supplement shortcomings, and a group of key enterprises with industrial ecological leadership and driving capabilities in subdivided fields will be cultivated.
Torrent single-use bioreactor
Single-use bioreactor is a necessary core equipment for biopharmaceutical enterprises. With the rapid growth of global biopharmaceuticals. Biopharmaceutical companies have an increasing demand for high-quality, low-cost, highly customized equipment and consumables. The market size of single-use biopharmaceutical devices is rapidly expanding.

JYSS BIO is the first high-tech enterprise in China to realize the localization of process technology for Single-use bioreactors and Single-use consumable . Its self-developed CUR torrent single-use bioreactor has international patents. At present, it has been successfully applied in many well-known domestic companies such as vaccines, gene and cell therapy, and antibody drugs.
JYSS BIO has a variety of self-developed and patented domestic membrane materials, Through the inspection of third parties and national institutions, it fully meets the requirements of domestic and international industry regulations. The biological film has reliable biosafety and production applicability, and can provide downstream manufacturers single-use bags with the same quality of imported membrane materials. At present, the biological film has achieved industrial application in many biopharmaceutical enterprises.
Application Case
CUR torrent bioreactors are widely used in suspension culture of cells (such as HEK293, CHO, etc.), and are suitable for different culture processes (such as batch culture, batch feeding, perfusion). Compared with the traditional stirred bioreactor, it can also obtain high or even higher cell density and viability. Some comparative data for cell cultures in CUR torrent bioreactors see below.
Case1:
The CHO cell perfusion culture process test was carried out on JYSS BIO CUR50 single-use bioreactor. The growth trend of cells in the torrent bioreactor CUR50 is consistent with that of the stirred bioreactor, and the density of viable cells is maintained at a higher density in the later stage. During the whole culture process, the viability rate is maintained at 98%+, which is better than that of the stirred bioreactor. The final protein expression on the torrent bioreactor was about 20% higher than that in the stirred bioreactor.
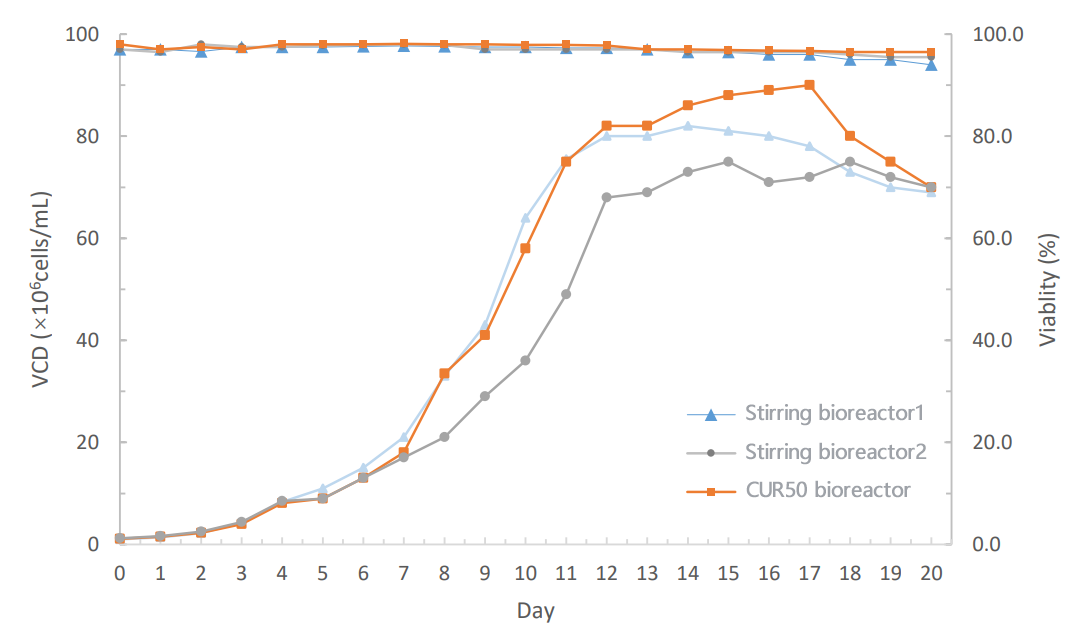
The cell growth trend is close to the scale-up of small scale and pilot scale
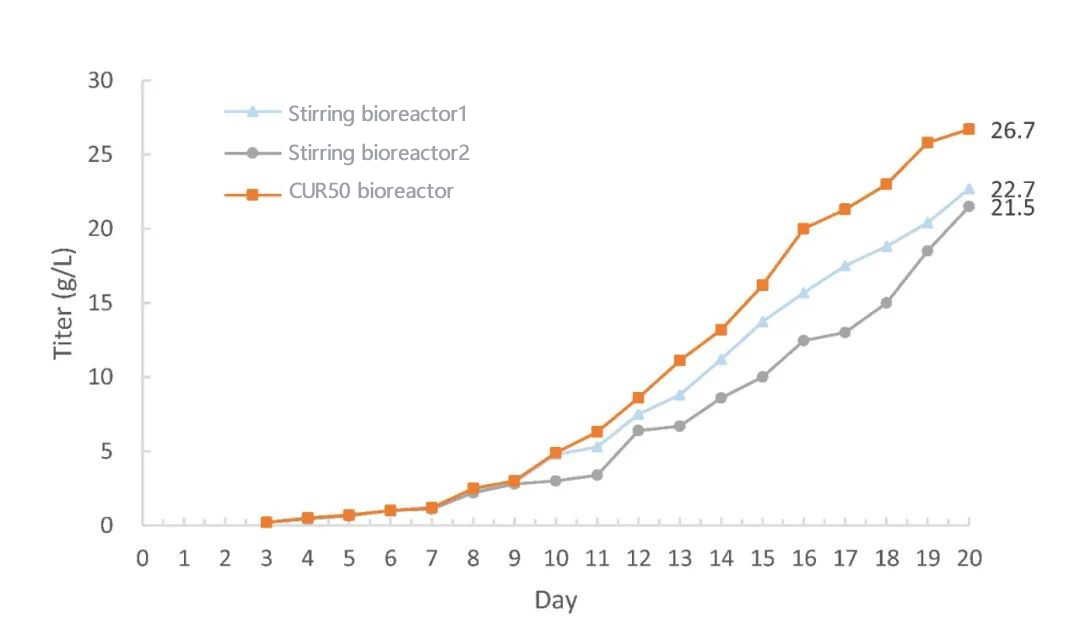
It is observed from the curve that the expression yield is higher than that of the previous small-scale and pilot-scale
Case 2:
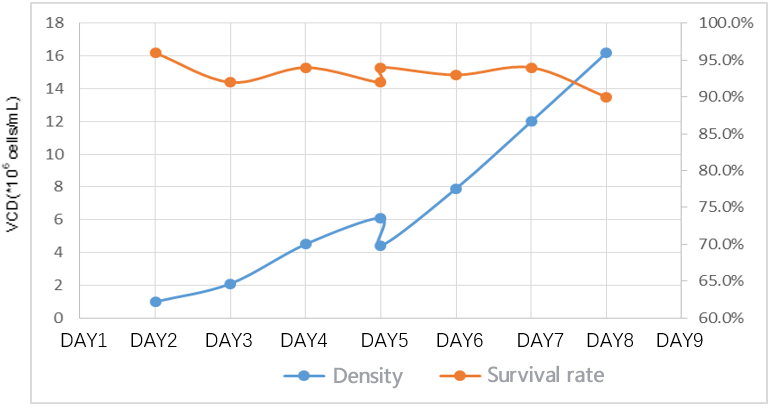
Lentiviral production was performed on HEK293T cell cultures on a CUR 5 single-use torrent bioreactor. The cell density was expanded from 1.0×10⁶ cells/ml to 6.0×10⁶ cells/ml within three days, and the survival rate was maintained at 90%+, which met the needs of transfection, and the survival rate after transfection was maintained at 90%. Virus titers were comparable to the shake-flask process.
Under the macro background of the rapid expansion of the single-use biotechnology market and the continuous acceleration of the pace of import substitution, JYSS BIO has always maintained sufficient strategic determination, and has a clear understanding of the company's current situation, development strategies, and external environment, and is firmly committed to firmly grasp the pace of development. With its own strong strength and the joint efforts of the whole industry chain, as soon as possible to achieve domestic replacement!